1The carbon and nitrogen cycle¶
1.1DONE: Vegetation phenology¶
1.1.1DONE: Classification of plant status¶
To accurately simulate seasonal ecosystem cycles it is essential to represent the biological life cycle of plants Lieth, 1974Piao et al., 2019. The timing of each of the plant phenological phases is dependent on a combination of physiological characteristics and climatic conditions. In ORCHIDEE, each PFT can progress through the following phases: establishment, bud presence, leaf onset, canopy presence, wood growth cessation (pre-senescence), senescence, dormancy, and death. The phenology models in ORCHIDEE control the timing of leaf onset and senescence. There are no leaf onset or senescence modules associated with evergreen ecosystems where leaf turnover is simply a function of climate and leaf age (equation 245).
1.1.2DONE: Leaf onset¶
There are four different modules to determine leaf onset (Table 4), where leaf onset is determined by moisture conditions, or temperature conditions (including the number of growing days, the occurrence of chilling days, and growing degree days), or both temperature and moisture conditions.
Table 4:The phenological modules used to determine leaf onset.
| Module | Leaf onset trigger | PFTs affected |
| NGD | Number of growing days | Boreal needleleaf deciduous forest |
| GDD-NCD | Growing degree days after cold period | Temperate broadleaf deciduous forest, boreal broadleaf deciduous forest |
| MOI | Moisture conditions | Tropical broadleaf raingreen forest |
| MOIGDD | Both temperature (GDD) and moisture conditions | Boreal natural C3 grassland, temperate natural C3 grassland, tropical natural C3 grassland, natural C4 grassland, C3 cropland, C4 cropland |
Within the number of growing days (NGD) module, which was originally proposed by Botta et al. (2000) for arctic and boreal biomes, the onset of leaves occurs when the number of growing days exceeds a PFT-specific critical value:
where refers to the number of growing days with a mean daily temperature above a threshold, 268.15 K in ORCHIDEE, calculated from the start of the midwinter (shortest day of the year) where, (days) represents a PFT-specific critical value (23.9 days for boreal needleleaf deciduous forests). To ensure it’s spring, temperatures must also be increasing:
where (K) and (K) represent the weekly and monthly mean temperatures, respectively.
The growing degree days - number of chilling days (GDD-NCD) module follows the general GDD model Chuine, 2000, which is designed to simulate the accumulation of warm temperatures in the spring needed for budburst and is a common model applied to satisfy temperature conditions for leaf onset to occur Hänninen & Kramer, 2007. As in the number of growing days model, temperatures must also be increasing (i.e., the weekly temperature must be greater than the monthly temperature - equation 239). GDD is calculated as a sum of the mean daily temperatures for days that have a mean daily temperature above a given threshold, starting from a given time point. Leaf onset occurs once the GDD value has crossed a defined threshold. In the GDD-NCD module the GDD is calculated from midwinter (as for the NGD module) but the general GDD module described above is further adapted to account for PFTs that require a period of cold temperatures to trigger budburst Orlandi et al., 2004 by modifying the GDD threshold to account for the number of chilling days. Thus, in the GDD-NCD module the start of the growing season occurs when the growing degree days since midwinter exceeds a calculated GDD threshold:
where (K) represents the growing degree days since midwinter and (K) is a negative exponential that accounts for the number of chilling days with an additional three fixed parameters, following Botta et al., 2000:
where (-), (-) and (-) are non PFT-specific model parameters that have been calibrated against satellite data, and are 964, 0.0058, and 12.8, respectively Botta et al., 2000. is the number of chilling days since midwinter, where chilling days are defined as the number of days with a mean daily temperature below a PFT-specific threshold (278.15 K and 273.15 K for temperate and boreal broadleaved deciduous forests, respectively) following Cannell & Smith (1986), Murray et al. (1989), and Chuine (2000).
Within the moisture module, the conditions determining the onset of leaf growth depend on either a soil moisture availability criterion (if hydraulic architecture is not used) or a vegetation stress proxy (if hydraulic architecture is used). This means that the growing season can begin if the last moisture minimum was a sufficiently long time ago (equation 242) and either the soil moisture availability (or vegetation stress proxy) is increasing (equation %s) or if the monthly moisture availability (or monthly vegetation stress proxy) is high enough (equation %s).
where (-) and (-) are proxies for weekly and monthly vegetation water stress, respectively, with values ranging from 0 (high moisture stress) to 1 (low moisture stress). (days) represents the number of days since the last moisture minimum, and (days) and (-) represent PFT-specific threshold parameters. For tropical broadleaf raingreen trees, and are 50 days and 1.0, respectively.
Vegetation water stress is calculated using the soil moisture availability criterion (essentially root profile weighted plant available relative soil moisture) that varies linearly from zero at wilting point to 1 at field capacity:
where (kg ) is the soil moisture of the -th (m) soil layer (liquid phase), (kg ) soil moisture of each layer at field capacity, (kg ) is the soil moisture of each layer at wilting point, and (-) is the normalized root mass/length fraction in each soil layer (0 - 1) Krinner et al., 2005. The moisture availability criterion (equation 242 - equation %s) correspond to Model 4b in Botta et al. (2000), which assumes that leaf onset in the dry tropics and semi-arid ecosystems occurs only after a certain amount of water has accumulated in the soil.
Within the moisture-growing degree day module (MOI-GDD), both the moisture conditions described above and a GDD threshold criterion must be met for leaf onset to occur. For the moisture conditions parameter is set to 35 days for grasses and 75 days for crops, and parameter is set to 0.98 for C3 grasses and 0.6 for C4 grasses and crops. The GDD test is similar to the general GDD module, described above, where the growing degree days since midwinter must exceed a calculated GDD threshold (i.e. equation 240). In this version of the GDD model represents the growing degree days calculated using days with a mean daily temperature above 278.15 K since midwinter and the (° C) is calculated using a second-degree polynomial using the long term mean annual air surface temperature MacBean et al., 2015:
where (° C), (° C) and (° C) represent GDD PFT-specific parameters and (° C) is the 3-year average 2 m air temperature. It is not possible to convert GDD between ° C and K and because these PFT parameters are used in ORCHIDEE in ° C, we have also written this relationship here in ° C. Where is 640 ° C, 400 ° C, 400 ° C, 450 ° C for c3 grasslands, c4 grasslands, c3 croplands, and c4 croplands. is 32.0 ° C, 0.0 ° C, 6.25 ° C, and 0.0 ° C for c3 grasslands, c4 grasslands, c3 croplands, and c4 croplands. is 0.1 ° C, 0.0 ° C, 0.0315 ° C, and 0.0 ° C for c3 grasslands, c4 grasslands, c3 croplands, and c4 croplands. As in the NGD and GDD-NCD models, temperatures must also be increasing (equation 239). An alternative to the GDD threshold is that the monthly mean temperature must be above a certain PFT dependent threshold (defined as 283.15 K for all PFTs). Finally, for c4 grasses the monthly mean temperature must also exceed a pre-set temperature of 295.15 K.
1.1.3DONE: Senescence¶
Leaf senescence occurs due to age, the cessation of the growing season, or the occurrence of crop harvests (Table 5). Leaf senescence can also trigger fruit, root, or stem senescence. In the occurrence of leaf and root senescence, the nitrogen stored in leaves and roots is transferred into the labile nitrogen pool. Evergreen species do not experience seasonal senescence. Furthermore, if there is very low leaf mass during senescence, all leaves are lost.
Table 5:The phenological modules used to determine leaf senescence.
| Senescence module | Senescence trigger | PFTs affected |
| Cold | Cold temperatures | Temperate broadleaf deciduous forest, boreal broadleaf deciduous forest, boreal needleleaf deciduous forest, |
| Dry | Water availability | Tropical broadleaf raingreen forest |
| Mixed | Photosynthesis to plant respiration ratio | Boreal natural c3 grassland, temperate natural c3 grassland, tropical natural c3 grassland, natural c4 grassland |
| Crop | Harvest | c3 cropland, c4 cropland |
| Age | Leaf age | All (except bare ground) |
Even if the meteorological conditions are favorable for leaf maintenance, plants, and in particular, evergreen trees, must renew their leaves simply because the old leaves become inefficient. Therefore, the old leaves of all PFTs fall off regardless of climatic conditions, following.
where is the carbon (g C m day) and nitrogen (g N m day) biomass of organs, (leaves, roots, fruits, and stems), of circumference class . is the fraction of carbon and nitrogen biomass lost, is the timestep (1 day), (days) is the age of the leaf mass in age class , and (days) is the location-specific leaf longevity, dependent on the long-term temperature:
where (days) is the PFT-specific leaf longevity and is 180 days for raingreen trees, summergreen trees, and grasses, 730 days for broad-leaved evergreen trees, 910 days for temperate needleleaf trees, 2000 days for boreal needleleaf evergreen trees, and 200 days for crops. (-), (-), and (-) are PFT specific parameters linking leaf longevity to the reference temperature. is 1.5 for grasses and crops, 2.0 for boreal needleleaf evergreen trees, and 1.0 for all other PFTs. is 0.365 for boreal needleleaf evergreen trees, and 0.75 for grasses and crops, and 1.0 for all other PFTs. is 120 for boreal needleleaf evergreen trees, 10 for grasses and crops, and 0 for all other PFTs. (K) is a PFT-specific reference temperature for the calculation of leaf longevity and is 298.15K for tropical trees, 293.15K for temperate broad-leaved evergreen trees, C4 grasses and crops; 288.15K for temperate needleleaf evergreen trees, temperate broad-leaved summergreen trees, C3 grasses and crops; and 278.15K for boreal trees.
This means that older leaves fall more frequently than younger leaves and therefore the leaf age distribution must be recalculated after leaf senescence. The fraction of biomass in each leaf age class is updated using the following equation:
where is the new leaf carbon and nitrogen fraction of leaf biomass in leaf age class , is the old leaf fraction, is the new total leaf biomass of circumference class , and is the old total leaf biomass. is the change in leaf carbon and nitrogen biomass in the considered class due to senescence.
Senescence at the end of the growing season, can be triggered by cooling temperatures, water availability limitations, or both. In the case of senescence at the end of the growing season, leaf senescence is only possible if leaves have reached a certain age (equation 248).
where (days) is the mean leaf age and (days) is the PFT-specific parameter for the minimum leaf age for senescence. is 90 days for tropical and temperate trees, 60 days for boreal trees, and 30 days for grasses and crops.
Leaf senescence due to cool temperatures at the end of the growing season is triggered by decreasing temperatures (equation 250) and the fall of the monthly air temperature below a threshold temperature (). The threshold temperature is defined using a second order polynomial of the long-term mean annual air surface temperature:
where (K) is the critical temperature for leaf senescence, (K) is the long term annual mean temperature and (-), (-) and (-) are PFT-specific empirical constants in the quadratic temperature senescence equation. and are 0.00375 and 0.1, respectively, for C3 grasses, and 0 for all other PFTs. is 16.0 for temperate broad-leaved summergreen, 14.0 for boreal broad-leaved summergreen, 13.0 for boreal needleleaf summergreen trees, 5.0 for grasses, 12.0 for C3 crops and 13.0 for C4 crops. In addition, temperature must be decreasing, following:
where (K) and (K) refer to the mean daily temperatures calculated over the past fortnight and the past month, respectively. Alternatively, leaf senescence due to cooling temperatures is also triggered if the ratio of plant respiration to photosynthesis is above a PFT-specific threshold (equation 251).
where () represents the mean of daily maintenance respiration and () represents the mean of daily photosynthesis calculated for the past week, respectively. represents the unitless PFT-specific minimum senescence ratio parameter and is set to 0.75 for C3 grasses, 0.89 for boreal needleleaf summergreen trees, 0.87 for boreal broad-leaved summergreen trees and 1.0 for all other PFTs.
Leaf senescence due to a lack of water availability is triggered by low soil moisture availability. As occurs in leaf onset (sect. 1.1.2), this can be calculated using either the soil moisture availability criterion, if hydraulic architecture is not used, or as a ratio between a proxy for stressed and unstressed ecosystem functioning if hydraulic architecture is used. Leaf senescence is triggered if either the weekly moisture availability (or vegetation stress) falls below a critical threshold () or the ratio of plant respiration to photosynthesis is sufficient (equation 251). The critical moisture availability is calculated as a function of last year’s soil moisture (or vegetation stress) minimum and maximum (or vegetation stress) using the following equation:
where is the critical moisture availability threshold, and are the minimum and maximum moisture availabilities over the past year, is a critical relative moisture availability for phenology, is the PFT-specific critical moisture availability for senescence, and is the PFT-specific relative soil moisture availability above which there is no humidity-related senescence. , and are 0.5, 0.3, and 0.8 for tropical broadleaf raingreen trees, respectively.
For grasses, senescence occurs if the ratio of plant respiration to photosynthesis is sufficient (equation 251).
For crops, the moment of leaf senescence equates to the actual harvest and the entire plant is made senescent. To identify the harvest moment for crops, a second order polynomial of the long-term mean annual air surface temperature is used:
where (days) represents the harvest time threshold, and (K) and (days) represent PFT-specific parameters. is 282K and is 160 days for all crop PFTs. For senescence to occur, the length of the growing season (number of days) must exceed . In addition, the growing degree days since the initiation of the growing season must be sufficient () where (° C) represents the growing degree days since the start of the growing season and (° C) represents the PFT-specific minimum number of growing degree days. is 2500 ° C for all crop PFTs.
For grasses, senescence at the end of the growing season is further extended to the whole plant (i.e. all carbon and nitrogen pools) except the carbohydrate reserve. For trees, senescence at the end of the growing season extends to the fine roots at the same rate as they lose their leaves. The rate of biomass loss of both fine roots and leaves (for trees) and leaves, roots, and fruits (for grasses) is prescribed through the equation:
where is the carbon and nitrogen biomass, is the fraction of carbon and nitrogen biomass lost, is the times step, (days) is the PFT-specific time constant of meteorological determined turnover parameter. Where is 25 days for boreal trees, 15 days for temperate broad-leaved summergreen trees, and 5 days for temperate needleleaf evergreen trees and grasses.
All remaining leaves are shed when leaf biomass falls too low (below a PFT-specific parameter). For deciduous trees not only leaves but also fruits and fine roots are lost at this moment.
1.2DONE: Photosynthesis¶
The assimilation rate (, ), stomatal conductance () and leaf intercellular partial pressure () are calculated for each leaf layer, by jointly solving a set of three equations (see below). Both for c3 and c4 plants, these three equations can be manipulated into the form of a standard cubic equation for , for which Yin & Struik (2009) proposed an analytical solution.
We implemented this analytical solution and the overall formalism for both c3 (Appendix B in Yin & Struik (2009)) and c4 photosynthesis (Appendix C in Yin & Struik (2009)).
1.2.1DONE: C3 photosynthesis¶
The Farquhar–von Caemmerer–Berry model farquhar1980 predicts the net assimilation rate as the minimum of the Rubisco-limited rate () and electron (e) transport-limited rate ():
These two parts of the model can be written in a single equation as:
where for the Rubisco-limited part and , for the e transport-limited part and . and are the carbon dioxide and oxygen partial pressures at the carboxylation sites, 1, is the compensation point in the absence of dark respiration (). This latter case assumes a purely linear e transport. is a constant representing the relative CO2/O2 specificity factor for Rubisco.
Following Farquhar & Wong (1984), the rate of e transport () is described as a non-rectangular hyperbolic function of irradiance:
where is the conversion efficiency of absorbed light into at strictly limiting light, is the maximum capacity of e transport, and is the convexity factor for response of to absorbed irradiance.
The leaf photosynthesis is coupled with the stomatal conductance as follows (Yin & Struik (2009)):
were is the residual stomatal conductance , is the dark respiration, is the mesophyll conductance and is a function describing the sensitivity of the stomata to either vapour pressure deficit () or leaf water potential (), depending on the selected drought scheme (see 1.3).
According to the first diffusion law of Fick, the transfer from (ambient partial pressure) to can be written as:
where is the leaf boundary-layer conductance.
The three equations 257, 259 and 260 with the three unknowns , and form the system to be solved for c3 plants.
1.2.2DONE: C4 photosynthesis¶
For c4 plants, CO2 is fixed by phosphoenolpyruvate (PEP) carboxylase in the mesophyll cell into a c4 compound, which then goes into a bundle-sheath cell, where it is decarboxylated to provide CO2 to Rubisco. The CO2 concentration in the bundle-sheath is high, thus preventing photorespiration.
where is the rate of PEP carboxylation, is the mitochondrial respiration in the mesophyll and fixed to and is the leakage of from the bundle-sheath to the mesophyll through the bundle-sheath conductance :
can be limited by the PEP carboxylation rate or the e transport rate. In the former case, we have:
where is the initial carboxylation efficiency of the PEP carboxylase, and is the maximum rate of PEP carboxylation at the saturated .
For the e transport-limited case for c4 plants, we use:
where is the rate of all e transport through photosystem II (PSII), is a fraction of electrons that follows a cyclic e transport (CET) around photosystem I (PSI), and is a fraction of electrons at PSI that follow a pseudocyclic e transport (PET). Both are not used for carbon reduction and photorespiration (which is negligible in c4 plants). Similarly, , a fraction of electrons at reduced plastoquinone that follow the Q-cycle, is not transferred to the plastocyanin. and are PFT-specific parameters. For we use:
where is the fraction of e transport rate partitioned to the mesophyll reactions, which is set at 0.4, and is the number of protons required to produce one ATP, which is set at 4.
As for c3 plants, the Rubisco-based assimilation of in c4 plants can be limited by either Rubisco activity or by e transport rate (equation 256) following the equivalent to equation 257:
where is the oxygen partial pressure in the bundle-sheath, , is the relative CO2/O2 specificity factor for Rubisco. For the Rubisco-limited rate, we have: , , , and for the e transport-limited rate: , , .
The oxygen partial pressures between intercellular air-space and bundle-sheath are linked through the following equation:
where is the fraction of PSII activity in the bundle-sheath and 0.047 accounts for the diffusivities for O2 and CO2 in water and their respective Henry constants.
To enable an analytical solution Yin & Struik, 2009 propose an equation for c4 stomatal conductance that is slightly different than for c3:
with , the level at leaf surface and the -based compensation point in the absence of .
The three equations 266, 268 and 262 with the three unknowns , and form the system to be solved for c4 plants.
1.2.3DONE: Parameter dependency on temperature, nitrogen and water¶
To account for the nitrogen dependency of we use the nitrogen use efficiency (NUE, defined as per leaf nitrogen content) as derived from observations by Kattge2009:
where is NUE scaled with (1.4.5, 321) and leaf efficiency weighted for the fraction of leaves in each age class. Leaf efficiency is scaled by the leaf age of each leaf age class.
Most of the photosynthetic parameters are temperature-dependent and their temperature dependency in ORCHIDEE follows either an Arrhenius function (, , , , ) or a modified Arrhenius function, accounting for a decrease at high temperatures (, , Medlyn et al. (2002)). In addition, for c3 plants, we account for an acclimation term on the two entropy factors that are involved in the temperature function for and and the ratio between and (kattge2007). These parameters are all function of the monthly temperature. Based on Kattge et al (2009), is directly inferred from (scaled with the temperature dependency of ) and the monthly temperature. There is no acclimation term on any of the parameters used for c4 plants.
The water limitation function is impacting photosynthesis through (, see 1.3), but it is also limiting , , and .
is scaled from leaf to canopy level by multiplying with (see 1.5.7). Apart from the , the other environmental parameters that drive the photosynthesis (temperature, ambient CO2, air humidity) are held constant along the canopy depth. We only account for a leaf nitrogen-related reduction of and along the canopy depth, depending on the fraction of absorbed light , as calculated in the radiation transfer scheme (see 1.3.2).
The photon flux density absorbed by leaf photosynthetic pigments at canopy level () is calculated as:
where is a conversion factor between units from to , and is the fraction (=0.5) of that is photosynthetically active.
1.3DONE: Maintenance respiration¶
Maintenance respiration refers to the carbon cost that occurs in living plant tissue to maintain a healthy and living state, e.g., resynthesis of enzymes and membrane lipids, maintenance of ion gradients across membranes, and acclimation to environmental changes Amthor, 1984. ORCHIDEE does not distinguish the underlying processes and instead uses a bulk approach ruimy1996 in which maintenance respiration of a specific PFT within a grid cell (; g C m s) is the sum of maintenance respiration of the different plant organs. It is calculated as a function of biomass modulated by the temperature and nitrogen content of the vegetative and reproductive plant organs:
where denotes the plant organ, denotes the total number of plant organs (see 1.4), (s) is a PFT-specific maintenance respiration coefficient prescribing the fraction of biomass that is lost during each time step and (g C m) is the stand level nitrogen biomass of plant organ . is set to zero for the above-ground and below-ground heartwood as well as the carbohydrate reserves as ORCHIDEE assumes that these organs have no maintenance respiration.
, is a temperature modulator (unitless) calculated for each plant organ as:
where (K) represents the mean air temperature above freezing over the 3 years preceding the time step under consideration. , , and are PFT-specific parameters. For the above-ground plant organs, the 2-meter air temperature (; K) is used for . For below-ground plant organs, represents the root-zone temperature (K) calculated as the vertical profile of soil temperature (see 1.7.1) weighted by the vertical profile of the root biomass (see 1.5.10). When drops below 273.15 K, is set to one, implying that maintenance respiration continues but levels off when freezing.
In equation 271, (unitless) is a modulator for the N concentration of organs and is calculated as:
where is a global parameter, is the reference carbon-to-nitrogen ratio for organ , i.e., 45 for leaves, 52 for roots and fruits, and 517 for the sapwood and carbohydrates reserves. (unitless) is the actual carbon-to-nitrogen ratio for organ which is truncated at 200 if the calculated carbon-to-nitrogen ratio exceeds this threshold.
The parameters , , , , but not , have been tuned at the PFT-level and at the global level to simulate biomass production efficiencies within the ranges observed by Vicca et al. (2012) and Campioli et al. (2015). Given that ORCHIDEE v4.2 does not simulate the carbon fluxes from the vegetation to the mycorrhizae, the latter is included in . The simulated maintenance respiration flux () should, therefore, not be compared against observed fluxes as their definitions are not identical.
1.4DONE: Allocation and growth respiration¶
1.4.1DONE: Allocatable carbon and nitrogen¶
Following bud burst, photosynthesis produces carbon compounds that are added to the labile carbon pool as long as the plant passed bud burst and is not in presenescence yet (see 1.1):
where (g C m) is the labile carbon pool at the current time step , (g C m) is the labile carbon pool at time step , (g C m s) is the photosynthetic carbon flux at the current time step, and is the length of the current time step which is one day given in seconds (s). If the plant is in presenescence or senescence (see 1.1), the photosynthetic flux is added to the reserve pool:
where and are the carbon contained in the reserve pool at time step and (g C m), respectively. The labile carbon pool is then split into an active and none-active pool. The size of the active pool is calculated as a function of plant phenology and temperature. If the plant passed bud break and is not in presenescence yet, the active part of the labile pool was formalized following Ryan (1991), Sitch et al. (2003), and Zaehle & Friend (2010):
where , and are PFT-specific parameters representing respectively a growth temperature coefficient, the reference temperature above which all carbon in the labile pool is allocated to growth, and the minimum temperature for allocation. The parameters , and were set such that no carbon is available for plant growth below -2 ° C, only 3 % of the labile pool is available for allocation at 0 ° C, and at 5 ° C, 100 % of the labile pool is available for allocation. is the 2-m air temperature at , and (g C m) is the total biomass increment at the current time step. If the plant passed presenescence, the active part of the labile pool is set to zero.
Prior to allocating, is used to sustain the maintenance respiration flux (; g C m s; see 1.3) up to a maximum of 100 % of . A first estimate of the growth respiration, i.e., the cost for producing new tissue excluding the carbon required to build the tissue itself Amthor, 1984, is set aside. This first estimate assumes that all the available carbon will be allocated and is calculated as:
where (g C m s) is the estimated growth respiration and is the fraction of carbon respired to grow one unit of carbon, which is set to 28 % McCree, 1974. Hence, the amount of carbon that is available for biomass growth is:
where is the length of the time step for the calculation of biomass allocation (see table 7). Once allocation is finalized, will be recalculated (section 1.4.4) to obtain the exact growth respiration for the actual biomass increment.
Whether all of the available carbon can be allocated or not depends on the nitrogen availability. The labile N pool is calculated as:
where and (g N m) are the labile nitrogen pools at and , respectively, and (g N m s) is the nitrogen uptake by the plants at the current time step. Subsequently, is adjusted for the available nitrogen after estimating the nitrogen that would be required to allocate entirely. This estimate assumes that all tissue has the carbon-to-nitrogen ratio of leaves:
where (unitless) is the actual carbon-to-nitrogen ratio of leaves for the PFT and grid cell under consideration and is the PFT-specific minimal carbon-to-nitrogen ratio for leaves. The maximal elasticity of the carbon-to-nitrogen ratio of leaves (unitless) is calculated as:
where is the PFT-specific maximal carbon-to-nitrogen ratio for leaves. Assuming all newly grown biomass has the actual carbon-to-nitrogen ratio of leaves, (g C m) represents the maximum amount of carbon that could be allocated at time step , given the available amount of nitrogen:
If there is enough nitrogen (thus ), can be allocated. If, however, there is not enough nitrogen to allocate the allocatable carbon entirely (thus ), the elasticity of the carbon-to-nitrogen ratio of leaves is used to adjust :
where is the maximal elasticity of foliage N concentrations and set to 0.25 and is the carbon that will be allocated either for restoring plant allometry or for plant growth as described in the following sections.
1.4.2DONE: Plant allometry¶
Biomass is allocated to leaves, roots, sapwood, heartwood, and fruits. Allocation to leaves, roots and wood respects the pipe-model theory Shinozaki et al., 1964 and thus assumes that producing one unit of leaf mass requires a proportional amount of sapwood to transport water from the roots to the leaves as well as a proportional fraction of roots to take up the water from the soil.
The scaling parameter between leaf and sapwood mass is derived from:
where (m) is the one-sided leaf area of an individual plant, (m) is the sapwood area of an individual plant, a calculated parameter (unitless) linking leaf area to sapwood area and, (unitless) is the water stress as calculated in section 1.1. Alternatively, leaf area can be written as a function of leaf mass () and the specific leaf area (; m g C):
Sapwood mass can be calculated from the sapwood area as follows:
where is the tree height (m) and is the sapwood density (g C m). Following substitution of equations 285 and 286 into equation 284, leaf mass can be written as a function of sapwood mass:
where
where is calculated as a function of the gap fraction as supported by site-level observations Simonin et al., 2006:
is the minimum observed leaf area to sapwood area ratio, is the maximum observed leaf area to sapwood area ratio and is the actual gap fraction of the canopy (see 1.5.8). By using the gap fraction as a driver of more carbon will be allocated to the leaves until canopy closure is reached.
Following Magnani et al. (2000), sapwood mass and root mass () relate as follows:
where the parameter (m) is calculated according to Magnani et al. (2000) (their equation (17)):
where is the hydraulic conductivity of roots (m kg s MPa), is the hydraulic conductivity of sapwood (m s MPa), is the longevity of sapwood (days) and is the root longevity (days). Following substitution of equation 290 into 287 and rearrangement, leaf mass can be written as a function of root mass:
where
Parameter values used in equations 284 to 292 are PFT-specific. The allometric relationships between the plant components and the hydraulic architecture of the plant are both based on the pipe-model theory, hence, the same parameter values for the hydraulic conductivity of the plant components, i.e., , , are used in their calculations (section 1.1).
1.4.3DONE: Restoring plant allometry¶
The different biomass pools have different turnover times (section 1.1), are affected differently by the phenological processes, and are managed differently. As a consequence, the actual biomass components may no longer respect the allometric relationships (see 1.4.2). Plant allometry is restored by making use of allometric relationships.
where , , and are, respectively, the leaf, sapwood, and root mass (g C plant) for circumference class following the allometric relationships. The carbon required to restore the allometric relationships is calculated as:
where , , and are the increment of the leaf, sapwood, and root biomass (g C plant) for circumference class and (g C plant) is the total biomass that is allocated to circumference class .
In ORCHIDEE v4.2, forests are modeled to have circumference classes with (plants m) identical trees in each circumference class. Hence, the allocatable biomass needs to be distributed across all individuals in circumference classes. Following the restoration of the allometric relationships, the biomass remaining to be allocated is calculated as:
Within a single time step, biomass is thus allocated until the allometic relationships are restored or is consumed. It may take several time steps, especially at the start of the growing season, to restore the allometric relationships.
1.4.4DONE: Plant growth¶
Once the allometric relationships are restored, the pipe-model can be used to allocate biomass to leaves, sapwood and roots during plant growth. Fruit allocation is calculated first as it does not follow allometric relationships:
where is a PFT-specific parameter representing the share of carbon that is allocated to the fruits. (g C m) is calculated at the PFT-level and then distributed over the circumference classes .
Equations 287 and 292 can be rewritten as:
and allometric relationship is used to describe the relationship between tree height and basal area:
where (m) is a grid cell specific parameter giving the tree height for a tree with a diameter of 1 meter (see 1.5.3), is a PFT-specific parameter for the relationship between basal area and tree height, and (m) is the basal area of an individual tree in circumference class . The change in height is then calculated as a function of and where (m) is the basal area increment:
The distribution of carbon across the circumference classes depends on the basal area of the model tree within each circumference class . Trees with a large basal area are expected to have a higher leaf area and photosynthesis and are, therefore, assigned more carbon for wood allocation than trees with a smaller basal area, according to the rule of Deleuze et al. (2004):
where and (m) are unknown. (m) is the circumference of the model tree in circumference class , is a fixed parameter (unitless), ranges between 2 and 3.5 in function of , and (m) is a calculated parameter. is a function of the diameter distribution of the stand at a given time step:
where and are parameters (unitless) and is the median circumference of the circumference classes for the PFT and grid cell under consideration.
Equations 293 and 297 to 302 need to be simultaneously solved. An iterative scheme was avoided by linearizing equation 300, which was found to be an acceptable numerical approximation as allocation is calculated at a daily time step, and hence, the changes in height are small and the relationship is locally linear:
where is the slope of the locally linearized equation 300 for all circumference classes and is calculated as:
where represents a typical increase in basal area for a single time step. Following linearization of equation 300, equations 293 and 297 to 304 can be substituted and rearranged to obtain a quadratic equation of which the positive root is used as a solution for . distributes photosynthates across the different circumference classes and as such controls the intra-species competition within a stand. thus depends on the total allocatable carbon and needs to be optimized at every time step. Once has been calculated, , , and are calculated. Unallocated carbon, typically in the order of 10 to 10, is added back to to preserve carbon.
For grasses and crops the height is calculated as a function of their biomass instead of function of basal area as is the case for forest. Likewise, a single circumference class is considered. Hence, intra-specific competition is not accounted for.
, and propose how the carbon should be allocated to jointly respect plant allometry and intra-specific competition in the case of forest PFTs and plant allometry in the case of grassland and cropland PFTs, supposing that there is enough nitrogen to sustain the proposed growth. Prior to allocating these estimates to their respective pools, it is checked whether there is sufficient labile nitrogen to sustain the proposed allocation. When nitrogen is limiting, the allocation will be linearly adjusted (see below). Given the non-linearity of the pipe-model (section 1.4.2), such adjustments should be avoided and when they occur should be kept small. ORCHIDEE v4.2 reduces the need to adjust the proposed allocation by estimating, at the start of the allocation process (section 1.4.1), the mass of carbon that can be allocated given the available nitrogen. Now that all the required information is available, the subsequent calculations finalize and refine the initial estimate.
Nitrogen allocation starts with the calculation of the allocation fractions:
where , , , and (unitless) are PFT-level fractions of the available carbon that are proposed to be allocated to the leaf, sapwood, root and fruit pools, respectively. The allocation within the sapwood pool is divided into above- and belowground sapwood, where the fraction allocated aboveground is given by the PFT-specific parameter . Following the pipe-model approach that states that increasing the leaf mass has to be complemented by an increase in sapwood and roots to sustain the hydraulic continuity of the plant, the nitrogen cost is calculated as the total nitrogen mass required to allocate 1 g of N to the leaves Zaehle & Friend, 2010Vuichard et al., 2019:
where is the carbon-to-nitrogen ratio of wood relative to the carbon-to-nitrogen ratio of leaves and set to 0.087, and is the carbon-to-nitrogen ratio of roots relative to the carbon-to-nitrogen ratio of leaves and set to 0.86. Given the actual carbon-to-nitrogen ratio of the leaves , the carbon that could be allocated (g C m) is calculated as:
In case there is not enough nitrogen to sustain the proposed biomass increments, i.e., is greater than ), will be reduced to meet the nitrogen availability by taking into account the elasticity of the carbon-to-nitrogen ration. The carbon that can be allocated , given the maximal carbon-to-nitrogen elasticity (unitless) and the available nitrogen (g N m), is calculated as:
In case there is sufficient nitrogen to sustain the proposed carbon allocation, does not need to be recalculated, but the carbon-to-nitrogen ratio will be decreased to use as much nitrogen as possible until the maximum elasticity of the carbon-to-nitrogen ratio is reached:
The nitrogen constraint is calculated at the PFT-level, and if nitrogen limitation requires a reduction in , , , and are all linearly reduced.
The biomass increments for both carbon and nitrogen are taken from the labile pools and allocated to their respective biomass pools:
Sapwood contains mostly physiologically active cells that transport water and minerals from the roots to the leaves through the stem Thomas, 2014. As the sapwood ages, the cells nearest to the center of the trunk accumulate chemical compounds which makes theses cells to give up their transport function to become what is known as heartwood Thomas, 2014. In ORCHIDEE v4.2 the carbon and nitrogen contained in the sapwood is turned over into heartwood with a PFT-specific time constant that represents the longevity of the sapwood. Contrary to the other turnover processes in ORCHIDEE v4.2, sapwood turnover is not added to the litter pool as the biomass is not lost, but converted to heartwood:
The initial estimate of the growth respiration (equation 277) can be recalculated now that the final allocation has been decided on:
The carbon that was set aside for growth respiration but not used, i.e., the difference between the initial estimate (; equation 277) and the final calculation (; equation 311) is moved back into the labile carbon pool:
1.4.5DONE: Labile and reserve pools¶
The target carbon mass for the labile pool is calculated as a function of the nitrogen biomass of the living plant organs and the actual 2-meter air temperature:
where is a PFT-specific parameter, (g N m) is the nitrogen mass of plant organ , and is the mean photosynthetic flux over the past week.
The target carbon mass for the reserve pool is calculated as a function of the phenology type of the PFT. For evergreen forests the target reserve pool is calculated as:
where and are PFT-specific parameters representing the share of the sapwood and roots, respectively, to the reserve pool, is the target mass for the reserve carbon pool, and (g C m) is the leaf carbon mass for a canopy that is in allometric balance with the sapwood carbon mass. For deciduous forest the target reserve pool is calculated as:
where is a PFT-specific parameter representing the share of the sapwood to the reserve pool. The value of also depends on the plant phenology. The value is higher when the plant is in (pre)senescence. For grassland PFTs the target reserve pool is calculated as:
ORCHIDEE v4.2 fills the reserve and labile pools at the same time but with different (arbitrary) speeds. In ORCHIDEE v4.2 there are two important differences between the reserve and the labile pool: (1) only the labile pool is used in the allocation but storing carbon and nitrogen in the labile pool comes with a carbon cost, i.e., maintenance respiration, and (2) the reserve pool comes without maintenance respiration but the carbon contained in it cannot be allocated. These differences imply that the plant should store as much carbon and nitrogen as needed for growth in its labile pool but no more than that to limit the respiration cost. This trade-off is formalized as:
For simplicity it was assumed that carbon can move freely between both pools. The optimal allocation to both pools can be calculated by solving equation 318 for .
As long as is below the target, is less than one, implying that the available carbon will be preferentially stored in the labile pool. Once the target has been reached, exceeds one, implying that the available carbon will be preferentially stored in the reserve pool.
Following carbon allocation to the reserve and labile pool, nitrogen is allocated:
ORCHIDEE v4.2 does not control the carbon-to-nitrogen ratio of the the reserve pool; even if nitrogen is limiting plant growth, carbon may accumulate in the reserve pool. Rather than respiring this excessive carbon Amthor, 2000Chambers et al., 2004, ORCHIDEE decreases its photosynthesis to reduce the further accumulation of carbon. Photosynthesis is reduced when a PFT has more labile and reserve carbon than the target mass of the labile and reserve pools. Reduced photosynthesis when reserve and labile carbon start to accumulate is justified by the observation that too much sugars in the leaves increases sap viscosity which in turn hampers the sap flow in the phloem. Viscosity can be reduced by closing the stomata and transpiring less of the sap flow from the xylem. By closing the stomata, photosynthesis will be reduced Hölttä et al., 2017. Because ORCHIDEE v4.2 does not calculate the phloem sap flow, turgor, or viscosity yet, a ratio is used to reduce the nitrogen use efficiency used in the calculation of photosynthesis (see 1.2). If the plant has less carbon in its labile and reserve pools than targeted, the nitrogen use efficiency is increased. Up and down regulation of nitrogen use efficiency is only accounted for between bud break and the start of (pre)senescence.
where (unitless) is a proxy for sugar loading at time step . The sugar loading used to regulate the nitrogen use efficiency is the average sugar loading over the last 7 days to avoid sudden changes in the nitrogen use efficiency.
1.5DONE: Vegetation characteristics¶
1.5.1DONE: Leaf age¶
ORCHIDEE v4.2 distinguishes four age classes () where the length (days) of an individual age class is PFT-specific and calculated as:
where is the PFT-specific, location specific critical leaf longevity (days) calculated as:
where is the parameter prescribing the longevity of a typical leaf or needle at the reference temperature (, K) for the PFT under consideration. , and are parameters to calculate the upper and lower boundaries of the critical leaf age. The parameter describes the temperature dependency of the critical leaf age in between its lower and upper boundary. The temperature dependency makes location specific in addition to , , , , and being PFT-specific. The temperature dependency enables simulating a range in longevity within in a single PFT.
Newly allocated leaf biomass () goes into the first age class:
where is the fraction of leaf mass in leaf age class and is thus the fraction of leaf mass in the first age class. The fractions for the youngest leaf age class is then calculated:
The fractions for the remaining leaf age classes are then calculated as:
Each time step, the age of the leaves () in age class increases with the number of days of the time step ():
The turnover between the leaf age classes is calculated as:
The fraction of the leaves in each age class is then updated:
The leaf age in each age class is updated from its previous value:
As there is no turnover in the last age class (), leaf age is updated as follows:
The leaf age fractions are then normalized to avoid numerical issues. Mean leaf age () is used in the calculation of photosynthesis (section 1.2) and senescence (section 1.1) and calculated as the mean of all age classes weighted by their mass fractions:
1.5.2DONE: Specific leaf area¶
The specific leaf area (m g) is needed to convert leaf mass, which is a prognostic variable in ORCHIDEE, to leaf area which is used in the calculation of, among others, photosynthesis, interception, albedo, and canopy cover. The specific leaf area of a PFT at a given location is calculated as a function of the leaf biomass Vuichard et al., 2019 to better account for the observed variation in specific leaf area Reich et al., 1999Poorter et al., 2012:
where is the PFT-specific extinction coefficient of the vertical nitrogen distribution in the canopy Dewar et al., 2012 and is the initial value for the PFT-specific specific leaf area. The user choose to use a fixed PFT-specific specific leaf area as an alternative to the dynamic approach described in this section.
1.5.3DONE: Tree height¶
In ORCHIDEE v4.2 the diameter and height of an individual tree are calculated from its tree mass by assuming: (1) a PFT-specific form factor to account for the fact that trunks are conical rather than cylindrical, (2) a PFT-specific wood density, and (3) a PFT-specific relationship between the diameter and height of the tree (i.e., equation 299). The relationship between tree diameter and height makes use of the parameter which represents the height of a tree with a diameter of 1 meter.
The calculation of as a function of precipitation is based on the principles laid out in Kempes et al. (2011). In that paper, transpiration () of an individual tree scales to tree height (equation 3 in Kempes et al. (2011)); likewise, the root radius of a tree scales to tree height (equation 4 in Kempes et al. (2011)), and the water available to an individual tree () is then calculated as a function of its root radius (equation 5 in Kempes et al. (2011)), shown as below:
The maximum tree height will be reached when all the water available to the tree is used for transpiration. Following substitution of equations 334 to %s, maximum tree height can be written as a function of precipitation () and is then calculated as:
where and are global parameters that were parameterized by fitting the annual precipitation sum from the CRUJRA climate forcing Harris et al., 2020Harris et al., 2014Kobayashi et al., 2015 to the GEDI-derived 75 % tree heights Potapov et al., 2021 for the year 2019. After assuming that the diameter of the trees that reached the 75 % percentile height was 0.5 m, the fitted , and observed 75 % height were used to recalculate for a tree with a diameter of 1.0 m in line with the definition of . represents the minimal value for and is used at the start of the simulation when the accumulated precipitation is still unrepresentative for the long-term precipitation of a given location, is the long-term precipitation represented by the annual precipitation over the last 3 years. Hence, due to its dependency on precipitation, is location-dependent rather than PFT-specific.
1.5.4DONE: Vegetation density¶
For croplands and grasslands, ORCHIDEE v4.2 defines an individual as all plants that occupy a square metre of land. The maximum density of a cropland or grassland in ORCHIDEE is therefore 10,000 individuals per hectare. The vegetation density of croplands, defined as the number of individuals per unit of area, is calculated as:
where d is the density of the cropland (ind m), and c is a specific parameter of the PFT that prescribes the density. The fixed density reflects the practice in agriculture to plant at the final density to avoid individual plant mortality before harvest. Although a temporally fixed density for croplands is justifiable, cropland PFTs in ORCHIDEE are global and only distinguish C3 and C4 plants. Maize grown in, for example, humid temperate and semi-arid climates have the same density in ORCHIDEE although the biomass of an individual may differ.
Unlike croplands, the actual density of grasslands is not fixed to a prescribed parameter, but instead is calculated. Grassland density is decreased based on both the reserve and labile carbon, and the adjustment is calculated daily when the reserve and labile carbon in the plant drop below their respective target values and this condition persists over a longer time period. The downwards adjusted density of a grassland is estimated in accordance with the available resources by redistributing the resources among a maximum number of individuals capable of surviving under the given environmental conditions. By decreasing the number of individuals and redistributing the labile and reserve carbon among the surviving plants, their chances of future survival increase. The new, decreased number of individuals is calculated as:
where M (gC ind), M (gC ind), and M (gC ind) are the carbon biomasses of the reserve, the labile pool, and the sum of all plant compartments of an individual. M (gC ind) and M (gC ind) refer to the target pools for reserve carbon and labile carbon, respectively, which are calculated as explained in Section 1.4.5. The subscript t and t+1 refer to the value before and after the density adjustment, hence, d (ind m) is the initial density, and d (ind m) is the downwards adjusted density.
Note that the carbon of other compartments, i.e., leaf, aboveground sapwood, root, and fruit in each individual remains constant when the number of individuals is decreased. The nitrogen content of each compartment in an individual is updated by multiplying the previous nitrogen content by the ratio of the previous density to the current density. A minimum density of 0.05 is set to avoid numerical errors. In ORCHIDEE, grassland densities is only decreased during the following phenological stages: pre-senescence, senescence, and dormancy (for a more detailed description of the phenological stages see Section 1.1.1).
When the carbon content in both reserve and labile pools exceeds the target, and there is carbon presents in the fruit pool, grass density will be increased. The carbon from the fruit, reserve, and labile pools will be used to create new individuals. After updating the number of individuals, the labile and reserve pools equal their target values:
where M (gC ind) is the biomass stored in the fruit pool. The density is only increased during the phenological stage labelled as growth (for a more detailed description of the phenological stages see Section 1.1.1). This approach for increasing grassland density reflects grass recruitment through asexual means, which is suited to treat perennial plants Blair et al., 2013. Note that the carbon of other compartments, i.e., leaf, aboveground sapwood and roots, in each individual remain constant during the density in increased. Nitrogen is treated using the same method as that applied to decreasing density.
In ORCHIDEE v4.2 the simulated grasslands density accounts for spatial and temporal dynamics by accounting for the carrying capacity of the location. For grasslands, the reserve and labile carbon pools are used as a proxy for the carrying capacity of a grid cell. When the density of a grassland increases, recruitment through vegetative reproduction is simulated, but the biomass of the new individuals is identical to the biomass of the already established individuals. Vegetation density of grasslands is an emerging property of the model, as there are no parameters describing the density.
For forests, the resource availability at a given location and PFT determines the maximum number of individuals (; m) that the site can support. Compared to a site with a low carrying capacity, sites with higher carrying capacities can maintain higher stand densities for the same quadratic mean diameter (). When the maximum carrying capacity is reached, individual trees die and the resources that become available as a result of this mortality event are used by the remaining trees. This process is known as self-thinning and formalized in the commonly observed self-thinning relationship Reineke, 1933Yoda et al., 1963.
where is is the PFT-specific exponent of the self-thinning relationship and expected to be between -0.66 Westoby, 1984Zeide, 1987 and -0.75 West et al., 1999. represents the carrying capacity for a given grid cell and PFT. ORCHIDEE v4.2 contains two approaches to simulate the carrying capacity. It is prescribed and thus fixed at the PFT-level, or the carrying capacity depends on the climatic conditions, available plant water, light, and nutrients Zeide, 1987Puettmann et al., 1993Zeide, 2005Pretzsch, 2006. In ORCHIDEE v4.2 the calculation of photosynthesis accounts for all these factors and could therefore be considered as a modifier for the carrying capacity.
During the spinup of the model, is calculated as:
where is the PFT-specific reference carrying capacity, is the PFT-specific pre-industrial reference photosynthesis, and (g C m s) is the pre-industrial reference photosynthesis for a given grid cell and PFT. is calculated as the decadal mean photosynthetic flux. As the purpose of a model spinup is to bring the carbon, nitrogen and water pools into equilibrium, the temporal dynamics are deliberately kept to the minimum. Therefore, the spinup establishes the spatial variation in . During transient, historical, and future simulations, the carrying capacity, and thus the stand density, evolves over time as the growing environment is changing due to global changes such as climate change and nitrogen deposition. The temporal dynamics in are then calculated as:
where is the mean photosynthesis over the previous decade. As an alternative to the calculated , a prescribed PFT-specific parameter can be used to calculate in equation 339. If a PFT-specific parameter is used, the carrying capacity is no longer a function of global changes.
The relative density index (, unitless) expresses the fraction of the actual density against the maximum stand density given the carrying capacity of the location and PFT. Although the concept of relative density is rooted in forest management Garcia, 2012, it can also be applied to unmanaged forest as it has been observed that the actual stand density of unmanaged forests is also below the maximum density based on their carrying capacity luyssaert2011. Nevertheless, the processes responsible for the reduction in stand density differ between managed and unmanaged forests. In managed forest, forestry and natural disturbances are the main drivers of the density reduction whereas in unmanaged forests competition between individual trees and natural disturbances are the main drivers.
ORCHIDEE v4.2 uses three different relative densities in its calculations: the actual (), the lower target (), and the upper target relative density (). If the actual relative density exceeds the upper target for relative density, the stand density () is reduced such that it matches the lower target for relative density (sections 1.6 and 1.4. The processes driving the reduction in stand density depend on the forest management strategy applied to the PFT and grid cell (sections 1.6 and 1.4). The relative densities are calculated as follows:
and are obtained from fitting a polynomial through five management-specific parameters. The five key parameters that define the targeted relative density are: (1) relative density of a newly planted stand, (2) the maximum relative density, (3) the relative density when the diameter reaches its maximum, (4) the largest tree diameter, and (5) diameter above which the initial relative density starts to increase. These parameters are defined for each management strategy in ORCHIDEE v4.2 as well as for unmanaged forests, and may vary by PFT. is the degree of the polynomial function which in turn depends on the forest management strategy (section 1.4. For unmanaged forest a two degree polynomial is used, for the other management strategies the polynomial is fitted with three degrees.
In ORCHIDEE v4.2 the forest density calculation accounts for spatial and temporal dynamics by accounting for the carrying capacity of the grid cell. For forests the carrying capacity is estimated from photosynthesis. Recruitment is calculated as a separate demographic process (Section 1.10.2) and recruits have a lower biomass than the already established vegetation in the stand. Although the parameter representing the carrying capacity is adjusted to the environmental conditions of each grid cell, the shape-parameter of the self-thinning relationship is held constant within the PFT for the length of the simulation. Stand density in forests, therefore, remains strongly guided by the self-thinning relationship.
1.5.5DONE: Circumference distribution¶
Whenever the model is set-up to run with more than one circumference class ( > 1), the total stand density based on the self-thinning relationship (section 1.5.4), has to be distributed over the different circumference classes. Equation 301 of the carbon and nitrogen allocation scheme requires that, at all times, more than one circumference class contains individuals. In ORCHIDEE v4.2, the number of circumference classes is fixed () to limit the memory use, but the boundaries and number of individuals in each circumference class are updated as the simulation proceeds. Given its flexibility and resemblance with observed circumference distributions pretzsch2009, a Weibull distribution is used to calculate the distribution in both managed and unmanaged forests.
The Weibull distribution is a continuous distribution implying that it also estimates occurrence probabilities of unrealistically large trees. In ORCHIDEE, these unrealistically large trees are avoided by introducing the parameter at which the Weibull is truncated. is prescribed and depends on the forest management strategy. The shape parameter (, unitless) of the Weibull distribution is calculated or prescribed depending on the forest management strategy:
where (m) is the quadratic mean stand diameter, is a prescribed parameter that depends on PFT, and (m) is the prescribed PFT-specific tree diameter above which a stand is harvested. is the same parameter as in section 1.4.2). The Weibull cumulative distribution function (; unitless) for circumference class is:
where (; m) is the center diameter of circumference class . This approach allows the circumference distribution to change dynamically, over the course of several decades, as the stand structure evolves. Under rotational even-aged management the distribution is expected to evolve, from a stand structure dominated by many small individuals to a structure centered around the diameter targeted for exploitation (). Under continuous cover forestry as well as for unmanaged stands, a structure with many small individuals and fewer large individuals is maintained through time. The circumference class distribution is updated daily after accounting for mortality (section 1.6.3), forestry (section 1.4), land cover change (section 1), and disturbances (section 1).
1.5.6DONE: Forest structure¶
The simulation of carbon and nitrogen cycling within ORCHIDEE is based on mass flow with a rigorous mass balance check, hence, the mass of the different components are among the prognostic variables. Structural characteristics are diagnostic variables that are, whenever needed, calculated from the mass of the relevant components. Structural characteristics of an individual tree can be calculated from its mass by assuming: (1) a PFT-specific form factor () to account for the fact that trunks are conical rather than cylindrical, (2) a PFT-specific wood density, and (3) a relationship between the diameter and height of the tree that depends on both precipitation and PFT (i.e., equation 299):
where (g C plant) is the stem mass of a tree in circumference class calculated as the sum of the sapwood (; g C plant) and heartwood mass (; g C plant). The parameter in equation %s is the wood density (g C m) and thus identical to in equation 286. The parameter in equations %s and %s are identical to in equation 299. Note that equation 299 can be derived from equation %s by substituting and rearranging equations %s and %s.
Substitution of equations 345 to %s and rearranging results in expressions of individual tree height and diameter as a function of stem mass:
The width of a tree ring is calculated daily for each circumference class as:
where (m) is the ring width for a tree in circumference class at , and and (m) are the basal areas of a tree in circumference class at and respectively.
1.5.7DONE: Canopy structure¶
Tree crowns are formalized as prolate spheroids which are ellipsoids rotated along their major which, in ORCHIDEE v4.2, is the vertical axis. The crown of an individual tree belonging to circumference class is thus characterized by a vertical (; m) and horizontal crown diameter (; m) which can be calculated from the tree height and thus depends on the stem mass of an individual tree:
where is a PFT-specific parameter that links the crown depth to the tree height and is a PFT-specific parameter that prescribed the relationship between the horizontal and vertical crown diameter. The crown diameters can then be used to calculate the crown volume (; m plant) and projected crown area (; m plant):
The crown volume at the stand level (; m m) is calculated as:
Because the parameters and are not density dependent, crown packing () may exceed unity. A crown packing of more than 1, indicates that the total crown volume at the stand level exceeds the total available space which is the volume confined by the maximum tree height in the stand and the surface area of the stand. If crown packing exceeds 1, the horizontal crown diameters in ORCHIDEE are until a crown packing of 0.74 is obtained. The target of 0.74 represents the maximum possible packing efficiency of equal spheres Hales, 1998. The adjusted crown diameters can then be calculated as:
Stand structure controls the amount of light that penetrates to a given depth in the canopy. Light penetration is used in the calculation of albedo (section 1.3), photosynthesis (section 1.2), allocation (section 1.4.2), and recruitment (section 1.10.2). Prior to calculating light penetration (section 1.5.8) the canopy is discretized in canopy layers and the leaf area within each layer is calculated.
For forest PFTs the height of the top of the canopy (; m) is given by equation 351 and the bottom (; m) is calculated as the difference between in equation 351 and the maximum vertical crown diameter (max()). The canopy height of grasses and crops, which is the height of the top of the canopy (), is assumed linear to leaf area by making use of a PFT-specific parameter. The height of the bottom of their canopy () is placed at an arbitrary 0.0001 m above the soil. The height of canopy layer (; m) is then calculated as:
For forest PFTs, the partial crown volume within each canopy layer is calculated for each of the layers that were defined at the stand level and each of the circumference classes. It is first checked whether level crosses the spheroid:
Level crosses the spheroid of circumference class if is greater or equal to or is less or equal to . If level crosses the spheroid, the calculations continues as:
where (m plant) represents the partial crown volume for circumference class at canopy level . Note that and have their centers at the origin and that the horizontal and vertical crown radius is used rather than the crown diameters. The partial crown volume at layer (, ) is calculated by summing over the circumference classes:
For grassland and cropland PFTs, the partial crown volume within each canopy layer is calculated for each of the layers that were defined at the stand level. Note that the canopy of grasslands and croplands is formalized as a cuboid contrary to the spheroid assumed for the forest PFTs. The crown volume within a canopy layer is:
where is the surface area of the PFT (m) which is set to 1 m in ORCHIDEE v4.2. For all vegetative PFTs, the leaf area within each canopy layer is then calculated as:
where is the foliage area volume density () which is assumed to be constant across the circumference classes and the canopy layers . Likewise to forest structure (section 1.5.6), canopy structure and characteristics are diagnostic variables that, whenever needed, are calculated from the mass of the relevant components.
1.5.8DONE: Gap fraction¶
The gap fraction, which is the basic information in calculating light penetration at different depths in the canopy, is calculated following the statistical approach of Haverd et al. (2012). Following minor adaptations, the implementation of Haverd et al. (2012) was incorporated in ORCHIDEE v4.2. The gap model represents the canopy by a statistical height distribution with varying crown sizes and stem diameters for each circumference class . The crown canopies are treated as spheroids containing homogeneously distributed single scatterers. Although this approach to model canopy gaps can explicitly include trunks, ORCHIDEE v4.2 excludes them, as the spectral parameters for our radiation model (section 1.3.2) are extracted from remote sensing data without distinguishing between leafy and woody masses.
The gap probability for trees (unitless) is calculated as a function of height (; m) and solar zenith angle (; radians):
where (unitless) is the projected crown area for an opaque canopy at height for solar angle and is calculated from the geometric relationships detailed in Appendix A in Haverd et al. (2012). (unitless) is the mean crown porosity at height for solar angle . The mean refers to the mean over the tree distribution over the circumference classes. is calculated as:
If falls between and , then the partial volume of a spheroid above height (, m plant), is calculated as the difference of the volume of the whole spheroid and the volume of a partial spheroid below height :
If exceeds the whole volume of the spheroid is above height and therefore is calculated as:
Finally (m) is the mean path length through all canopy levels above height for solar angle for circumference class and is calculated as:
Since a tree crown is not opaque, the crown porosity is calculated by considering the projection of the leaf area in the direction of the incoming light. A spherical leaf area distribution is assumed which makes the projected leaf area in the direction of the incoming light constant and equal to 0.5 Pinty et al., 2006. The crown porosity is then calculated as:
where is a PFT-specific parameter prescribing the leaf clumping at the level of the plant shoots. By considering the stand density in each circumference class , stand level porosity which is used in equation 358, is calculated as:
As ORCHIDEE also simulates crops, grasses, and bare soil, the calculation of was adjusted for these PFTs as well. For grasses and crops, the same formulation is used:
where (unitless) is the total amount of LAI above height , and accounts for the fact that grasses and crops are treated as homogeneous blocks of vegetation with no internal structure. For bare soil, there is no vegetation to intercept radiation, and therefore is always unity. Depending on the calculations, is either used for a specific height and solar angle (e.g., albedo in section 1.3.2) or is integrated over all layers (e.g. light reaching the forest floor in section 1.10.2).
1.5.9DONE: Effective leaf area¶
The effective LAI for canopy layer with its bottom at height for solar angle is computed to be used with the two-stream albedo model (section 1.3.2) by using equation 25 in Pinty (2004):
Calculation of (unitless) is a computationally expensive part of ORCHIDEE v4.2, due to the loops over grid cells, PFTs, circumference classes, canopy levels, and solar angles in addition to using trigonometric functions. Moreover, a value for is needed every time step so, up to 48 times a day. Computational costs were reduced by calculating for eight solar angles (i.e., 5 °, 15 °, 25 °, 35 °, 45 °, 55 °, 65 ° and 75 °) exactly, and then fitting a function (i.e., ) to preset solar angles to predict the value of at any solar angle. A least square method is used to fit the parameters , , , , and . This method works well for low solar zenith angles, which are the most important since they will have the most incoming solar radiation.
1.5.10DONE: Root distribution¶
ORCHIDEE v4.2 calculates two vertical root profiles: a structural and a functional. Each root profile gives the share of the root biomass in each soil layer. The structural root profile is constant over time and is used in processes where the depth of the roots is one of the drivers, i.e., soil water infiltration along roots (section 1.7), input of soil carbon and nitrogen at depth due to the turnover of roots (section 1.8.4), maintenance respiration (section 1.3), and resistance to wind throw (section 1.2). The functional root profile changes over time and is used in processes where the vertical profile roots activity, rather than the vertical profile of root mass, is considered an important driver, i.e., transpiration (6).
The maximum rooting depth () is calculated by considering two constraints: (1) roots do not extend into frozen soils, and (2) crops are assumed to root to 0.8 m, grasslands are assumed to root no deeper than 1 m, and forests are assumed to root down to 2 m. Note that in ORCHIDEE v4.2 the maximum rooting depth does not depend on the root biomass, hence, plants root down to their maximum rooting depth from the start of the simulation.
The structural root profile is calculated as an exponentially decreasing root mass with depth where the shape of the profile is determined by the PFT-specific parameter Rosnay, 1999. The total surface area under an exponential curve between (m) and (m) is calculated:
The structural root profile (unitless) is then calculated as:
where (m) is the depth of the layer and (m) is the depth of the previous layer. The calculation is repeated for all layers between and .
The functional root profile gives the share of the root biomass in each soil layer based on the soil water in each layer. This results in a dynamic root profile that is changing over time. The total active root mass is calculated as:
The functional root profile (unitless) is then calculated as:
1.6DONE: Natural mortality¶
1.6.1DONE: Defining plant mortality¶
ORCHIDEE v4.2 distinguishes between plant and PFT mortality. Plant mortality is defined as an event in which part of the individuals within a PFT die without changing the ground area of this PFT. The causes of natural plant mortality accounted for in ORCHIDEE are described in section 1.6.2 and in section 1.4 for anthropogenic plant mortality. ORCHIDEE defines PFT mortality as an event in which all individuals within a PFT die without changing the ground area of that PFT. The causes of natural PFT mortality accounted for in ORCHIDEE are described in section 1.6.3. Anthropogenic causes of the mortality of forest PFTs is described in section and for agricultural PFTs in section 1.3). Events in which all or part of the individuals of a PFT die and the ground area of the PFT changes also distinguish natural and anthropogenic causes. The natural causes of stand replacing disturbances are described in section 1.6. The anthropogenic process ORCHIDEE accounts for and that results in an area change is land cover change which is described in section 1.1).
1.6.2DONE: Natural plant mortality¶
Natural plant mortality, i.e., an event in which part of the individuals within a PFT die without changing the ground area, is considered when the PFT has not yet been marked for killing due to PFT mortality (section 1.6.3. Two natural sources of plant mortality are considered: (1) self-thinning due to resource competition in forests, and (2) background mortality due to environmental stressors. The daily calculation of the self-thinning mortality for unmanaged forests starts with calculating three relative densities, i.e., the actual (), the lower target (), and the upper target relative densities (), according to equations 342 to %s. If the actual relative density () is less than its upper target (), the actual stand density is within the carrying capacity of the site and is, therefore, maintained. The carrying capacity is exceeded if the actual relative density exceeds its upper target (). A reduction in stand density () is then calculated such that the actual relative density matches its lower target for ():
where is the stand density (m) that has to be removed to reach the lower target for relative density and is the maximum stand density the site can support given the actual quadratic mean diameter of the stand. Since in ORCHIDEE the number of trees are given per unit area, the total number of trees and stand density are identical and thus interchangeable. The use of circumference classes adds realism to the ORCHIDEE v4.2 simulations, but it raises the questions which trees should be targeted by self-thinning mortality? For self-thinning, the mortality distribution () over the circumference classes is considered to follow the diameter distribution (section 1.5.5). The targeted mortality in each diameter class is then calculated as:
where (m) is the stand density that is killed in each circumference class. If self-thinning mortality occurs, environmental mortality is not accounted for. If there is no self-thinning, environmental mortality is accounted for. The model user can choose from two approaches to calculate mortality from environmental stress. In the first default approach the environmental mortality is considered constant:
where is the total biomass that needs to be killed, the biomass of trees in circumference class , the stand density in circumference class , and is the PFT-specific maximum age (days) for the PFT under consideration.
In the second approach the daily mortality from environmental stress is a function of the productivity of the PFT:
where is the maximum mortality, is the reference mortality and is minimum mortality. , , and are in year and are all PFT-specific. (g C m s) is the 3-year mean net primary production. is the total biomass that needs to be killed but ORCHIDEE v4.2 requires the stand density that has to be killed in each circumference class (; m). Where for self-thinning mortality, mortality was assumed proportional to the stand density in each circumference class, environmental stress was considered to preferentially affect the larger trees, being more prone to cavitation, wind damage, lightening, and heart rot. While killing the larger trees also trees in the other diameter classes were killed following Bellassen et al., 2010:
where is the death distribution factor, which is the factor by which the smallest and largest circumference classes differ e.g., a of 10 means that the largest circumference class will lose ten times as much biomass as the smallest circumference class as a result of the mortality.
ORCHIDEE v4.2 accounts already for several sources of mortality, i.e., forestry for managed forests, self-thinning for unmanaged forests, storms and insects for forests and fire for all PFTs and will be further developed to include even more sources. The end goal of these developments is to replace the pure empirical environmental mortality by a more mechanistic approach towards mortality. This is an ongoing process Bellassen et al., 2010Naudts et al., 2015Naudts et al., 2016Luyssaert et al., 2018Chen et al., 2018Marie et al., 2023 and until this goal has been reached, environmental mortality is kept as a fail-safe approach but the value of is increased every time a new source of mortality is added to the model. Likewise, with the addition of new sources of mortality, the current self-thinning mortality is evolving from a poorly defined mixture of sources of mortality towards pure competition driven mortality.
1.6.3DONE: Natural PFT mortality¶
PFT mortality. i.e., an event in which all individuals within a PFT die without changing the ground area of that PFT, occurs in two cases: carbon starvation and density-driven mortality. In both cases, all individuals in the PFT are marked for killing and PFT ground area does not change.
If at the end of the day, there is no carbon available in the carbohydrate reserve, leaf and labile pools, all individuals in the PFT will be marked for killing. This condition is the result of carbon starvation and typically takes 3 to 5 years to develop in ORCHIDEE v4.2. In this situation, the plants are not able to grow new leaves which are essential to assimilate new carbon from the atmosphere to initiate plant recovery.
In case of high intensity management or natural disturbances, the stand density could become so low that unrealistically large tree diameter would be simulated. In ORCHIDEE v4.2 this situation is prevented through density-driven PFT mortality Bellassen et al. (2010). Density-driven PFT mortality is not an ecological process but a fail-safe approach to keep stand density and tree diameter within observed boundaries.
1.6.4DONE: Moving biomass to litter pools¶
For each circumference class either none, part, or all of the individuals are marked for killing (; m) (sections 1.6.3 and 1.6.2). Subsequently, the biomass of the individuals that were marked for killing, is moved into the appropriate biomass pools. This section describes mortality in unmanaged PFTs only but the mortality could be caused by: carbon starvation (section 1.6.3), density-driven (section 1.6.3), self-thinning (section 1.6.2), environmental stress 1.6.2), bark beetles 1.3), or wind storms 1.2). Trees marked for killing are moved into the litter pool and the stand density is updated:
where and are the litter mass at respectively time step and for a single PFT within a grid cell, and (g plant) is the plant mass in circumference class . This calculation is repeated for the carbon and nitrogen biomass.
For grasslands and croplands fewer sources of mortality are accounted for in ORCHIDEE v4.2 compared to forest. Moreover, the density of a grassland or cropland is constant in ORCHIDEE. When partial mortality occurs, the individual plant mass is adjusted rather than the number of individuals as is the case in equation %s.
1.7DONE: Litter decomposition¶
1.7.1DONE: Adding biomass to the litter pools¶
The litter is formalized as four pools: a metabolic, structural, woody, and a snag, i.e., standing dead trees, pool. These four pools further distinguish an above-ground and a below-ground component with the exception of the snags that only have an aboveground pool. The metabolic litter is the labile or rapidly decomposing fraction of the litter, where the structural litter is the more resistant and, therefore, slowly decomposing fraction. This discretisation thus accounts for differences in the decomposition rates between cytoplasmic compounds of plant cells, and cell walls Corbeels, 2001. The woody pool represents the coarse woody debris, even slower decomposing than the structural pool Enrong et al., 2006, and the snag pool containing the dead standing trees, and assumed to be the slowest pool to decompose Enrong et al., 2006. In ORCHIDEE v4.2 the snag pool is optional. When the snag pool is not accounted for, all dead woody biomass is transferred to the woody litter pool.
The litter pools receive dead biomass as an input: each of the nine dead biomass pool is moved to one or two specific litter pools: the share of leaves, fruit and root biomass that is moved to the metabolic pool is calculated as:
where is the fraction of the input biomass of organ that goes into the metabolic pool, with being fruit, leaf or root biomass, is a reference fraction along with an empirical coefficient. (g lignin g C) is the lignin to carbon ratio of the organ of the input biomass and is the carbon-to-nitrogen ratio of the input biomass. The remainder, i.e., 1-, is added to the structural pool. Following mortality, leaves and fruit biomass is entirely added to the above-ground litter pool whereas the mass of dead roots is added to the below-ground litter. The labile and reserve biomass are moved to the above-ground metabolic litter pool. Sixty-eight percent of the heartwood and sapwood biomass that dies is moved into the woody litter pool, the remaining 32 % into the snag litter pool but this proportion needs to be refined as it is based on observed snag proportions Rahman et al., 2008Aakala, 2010Motta et al., 2006Sippola et al., 1998, which also results from the decay rates of the pools. Above-ground and below-ground heartwood and sapwood are already distinguished at the plant level, so following mortality, these mass components are moved in the matching above-ground and below-ground wood litter pools.
The lignin content of the different litter pools is calculated according to:
where (g C m s) the input of lignin to the litter pool , is the PFT-specific lignin to carbon ratio of the biomass pool , the number of plant organs and thus biomass pools, and (g C m s) the carbon input from plant organ into litter pool .
When a PFT is fertilized with manure, the nitrogen contained in manure is read from a fertilisation map (section 1.9) and added to the soil mineral nitrogen pools. The carbon contained in the manure is calculated from the prescribed carbon to nitrogen ratio of manure and added in the metabolic above-ground litter pool. By doing so, we mimic the application of manure on top soil by farmers that brings C to soil and since manure is considered to be a labile material with high turnover rates, we added it into the metabolic above ground pool. (ADD RATIONALE TO DO DO SO)
1.7.2DONE: Dynamics of the litter pools¶
The dynamics of the litter pools is based on the CENTURY first order decay model Parton et al., 1993 extended by coarse woody debris and snags, which follow similar dynamics Boulanger2011. Snags start as standing dead wood but due to decay at their stem base near the ground, snags will fall well before they are decomposed Hararuk2020. During snag fall, the mass of the snag is transferred to the above-ground woody litter pool, according to a fall rate with no control of moisture or lignin content (contrarily to the litter pools decay). Each of the pools are divided between an above and a below pool, which decompose in the same way with exception to the decomposition moderator functions (accounting for temperature, decomposer presence, and humidity effects). The litter dynamics are described as:
where (g C m or g N m) the carbon and nitrogen mass of the litter pool . In equations (379), eq:struc_litter, eq:wood_litter, eq:snag_litter stands respectively for metabolic, structural, woody and snag. (g C m s or g N m s) are the inputs, the decomposition rates, the snags fall rate, and the rate modifiers respectively accounting for the effect of soil moisture and soil temperature on decomposition. is a modifier that represents the moderating effect on decomposition of the lignin content in the litter pool (; g lignin g C). The rate modifiers follow the equations below:
j is above or below: when j is above is the humidity relative to field capacity, and is , the surface temperature, and for j being below is and is , the relative humidity and temperature averaged over soil layers and accounting for the decomposers’ presence, both calculated according to (386). , , , , , are empirical parameters which values can be seen in equations (385), eq:som_decomp_moisture_modifier which are the same as the equations for the below litter pools.
1.7.3DONE: Heterotrophic respiration from litter decomposition¶
When litter is decomposed, carbon dioxide is released in form of heterotrophic respiration. Heterotrophic respiration is calculated as a fraction of the carbon fluxes going from the litter to the soil carbon pools (section 1.8.1).
where (g C m s) is the heterotrophic respiration in the pools which involve a lignin fraction thus all but the metabolic litter pool, is the heterotrophic respiration in the metabolic litter, the number of soil pools, the index of the soil carbon pools, the carbon from the decayed litter pool , the lignin content in g lignin g C of the litter pool , the fraction of the decayed litter going to the soil (and not the atmosphere via heterotrophic respiration) and (g C m s) is the total heterotrophic respiration from the litter decay. All nitrogen released during the decay of the litter pools goes initially into the mineral nitrogen pool of the soil, where it is available for plant uptake, or other nitrogen processes (section 1.8.5).
1.8Soil carbon decomposition, nitrogen transformations, and heterotrophic respiration¶
The representation of soil carbon in ORCHIDEE is based on the CENTURY model parton1988. In each grid cell and PFT, the soil carbon is split between surface, active, slow, and passive pools. The surface pool has been added in ORCHIDEE to [increase the availability of nitrogen for plants??]. Optionally (see Sec. 1.8.4) the pools can be further split into depth layers, allowing for the representation of the vertical diffusion of organic matter due to cryo- and bioturbation.
The representation of soil nitrogen in ORCHIDEE is based on...
1.8.1DONE: Adding litter to soil carbon and nitrogen pools¶

Figure 13:Partitioning of decomposed litter between the surface, active, and slow soil organic carbon pools. The passive soil organic carbon pool (not shown here) does not receive litter, but only decomposed organic matter from the other pools.
DSG:
For respiration you separate litter and SOC although its the same shape of equations. (FK: TODO, will see what we can do.)
Using f for a parameter will confuse readers. (FK: f is both a fraction and a parameter, so both ’f’ and ’c’ would be appropriate. I agree with whoever made the choice of ’f’, the fraction-nature seems more important to me here than the fact that it’s a constant.)
In each time step, the mass of carbon in the decomposed litter is split between the surface, active, and slow soil organic carbon pools as shown in Fig. 13. The fraction of decomposed carbon from litter pool () that enters the soil organic matter pool is controlled by the parameter . In addition to this parameter, the distribution of decomposed structural, woody, and snag litter takes into account the PFT-dependent lignin content and allocates the non-lignin fraction to the active and surface pools and the lignin fraction to the slow pool. The resulting input fluxes of soil organic carbon pools are
1.8.2DONE: Dynamics of soil carbon and nitrogen pools¶
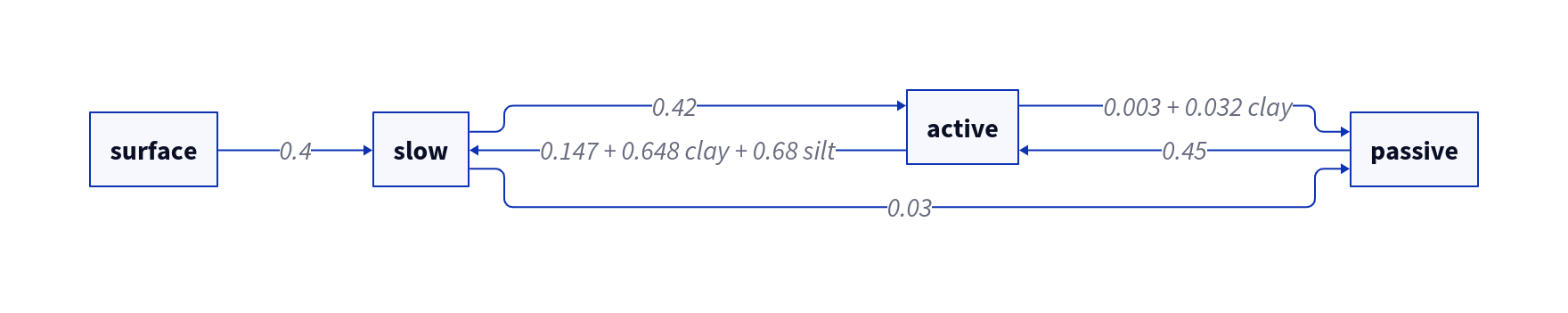
Figure 14:Flow of soil organic carbon between the pools. Each connection shows the fraction of the decomposed organic matter in the source pool that enters the destination pool. In all cases, the remaining decomposed organic matter is respired. Clay and silt refer to soil textural fractions (0–1).
ORCHIDEE keeps track of the concentrations (in ) of element (carbon or nitrogen) in pool in each grid cell and PFT (indices suppressed). In each time step, these concentrations are updated according to the following equation combining the litter input flux , the decomposition output flux , and fluxes between the pools,
where the last term is the flux from pool to pool , calculated as a fraction of the decompositon flux of pool . The default values of the fractions are shown in Fig. 14.
The decomposition flux for pool is calculated assuming linear kinetics with turnover time , which is modified to account for soil temperature, moisture, and clay content, as well as for tillage in crop PFTs,
The default turnover times are , , , and .
The temperature and moisture modifiers parton1988,
depend on and which are the temperature and volumetric water content of the current soil layer (if vertical dicretization of soil organic matter is activated) or averages over the soil profile. The averaging is done by integrating temperature or moisture over depth with a weighing factor reflecting the assumed concentration of decomposers close to the surface. This weighing factor is exponential with a characteristic depth of , equal to 0.2 m by default. By assuming the moisture is uniform in each soil layer, the integration for soil moisture gives
where is the soil moisture in layer , is the depth of the lower boundary of layer as used in the soil hydrology calculations (with being the depth of the upper bondary of layer 1). The average temperature is calculated analogously.
As in the original CENTURY model parton1988, the decomposition rate in Eq. (384) is decreased in proportion to the clay content of soil to represent the slowing down of decomposition due to association of organic matter with minerals. The clay modifier is equal to 1 for all but the active pool, for which it is determined by the clay content (0–1),
Finally, the decomposition rate is increased to account for the effect of tillage in crops. This modifier is based on [ref??] and given by
1.8.3DONE: Heterotrophic respiration from soil carbon decomposition¶
Heterotrophic respiration from soil (F) is obtained by summing the fractions of decomposition fluxes which do not enter any other soil organic carbon pool,
All nitrogen released during the decay of the som pools goes into the mineral nitrogen pool of the soil, where it is available for plant uptake, or other nitrogen processes (section 1.8.5). Please note that when soil organic matter is discretized the mineral N pool is not therefore all the minerazlied N is going into a single mineral N pool.
1.8.4DONE: Vertically discretized carbon and nitrogen pools¶
When soil organic matter discretization is activated, the active, slow, and passive pools are divided into depth layers identical to the ones used in the soil thermodynamics calculations. In each layer of pool , ORCHIDEE keeps track of the volumetric concentration (in ) of element (carbon or nitrogen). When soil organic matter discretization is activated, the surface soil organic matter pool is not used.
The areal (per ) litter input flux of element to pool is split into volumetric (per m³) fluxes entering layer following a dimensionless profile ,
where is the depth of the lower boundary of layer of soil thermodynamics and . The profile is normalized to sum to 1 and extends down to a grid-cell- and PFT-dependent depth . Above that depth, it is proportional to the integral of a piecewise constant exponential with a grid-cell- and PFT-dependent characteristic depth , giving
By default (runtime flag new_carbinput_intdepzlit set to false), and are both be set to last year’s maximum active-layer thickness or to , whichever is larger. In the new approach (new_carbinput_intdepzlit set to true), is set to the maximum depths root occur at (), as calculated by the soil hydrology scheme. At the same time, is set to the fine rooting depth of a given PFT or to a value proportional to the last year’s maximum active-layer thickness, whichever is smaller.
Following the distribution of litter, the decomposition of soil organic matter proceeds independently in each layer using the same formulation as the bulk soil organic matter dynamics described previously. Following decomposition, vertical transport of soil organic matter by cryoturbation or bioturbation is simulated. Both processes are assumed to follow Fick’s law of diffusion and differ only in the value of the depth-dependent diffusion constant . That is, for each element and pool the vertical soil organic matter flux is
Cryoturbation takes place in grid cells and PFT tiles in which last year’s maximum active layer thickness is larger than or equal to a parameter set to by default. In tiles where last year’s maximum active layer thickness is larger than or equal to a parameter set to by default, bioturbation takes place instead.
There are 6 alternative parametrizations of the depth-dependent cryoturbation diffusion constant. In the default (or old) parametrization and in the new parametrizations numbered 1, 2, 4, and 5, the cryoturbation diffusion constant is equal to a parameter down to the layer corresponding to last year’s maximum active layer thickness . In the layers below, the diffusion constant decreases in a way that depends on the parametrization. In the old parametrization, the diffusion constant in the layers immediately below is equal to, respectively, , , and 0 all the way down to the bottom of the soil column. In the new parametrizations numbered 1, 4, and 5, the diffusion constant decays linearly reaching zero either at (parametrization 1), at (parametrization 4), or at (parameterization 5). In parametrizations 2 and 3, the diffusion constant decays exponentially with a characteristic depth of . The decay starts below in parametrization 2 and at the soil surface in parametrzation 3.
The bioturbation diffusion constant has a unique parametrization. It is equal to a parameter down to a constant depth , equal to by default, and 0 below.
The diffusion equation (392) is solved using the same (finite-difference, implicit) numerical scheme as used for the soil heat transport. The scheme is described in detail by Li (1990) and by Hourdin (1992) (Annex A).
1.8.5Nitrogen fluxes from soil dynamics¶
Where should we describe the impose_cn option? Here? or in allocation where it is most impactful or in phenology where it is also applied? I described impose_cn = n as "a dynamic nitrogen cycle allowing nitrogen limitation" in the description of the model configuration (section 1). I have not yet describe impose cn = y but in line with the above it would become "a dynamic nitrogen cycle preventing (or avoiding?) nitrogen limitation". Feel free to change the wording. If changed, please also change in section 1.
Mineral nitrogen is represented by five pools: ammonium (NH / NH), nitrate (NO), nitrogen oxides (NO), nitrous oxide (NO) and dinitrogen (N) soil pools. The fate of mineral N in the five pools is controlled by the internal N flows associated with nitrification (the oxidation of NH / NH in NO) and denitrification (the reduction of NO up to the production of N{) processes and by incoming and outgoing mineral fluxes. Incoming fluxes which are accounted for in ORCHIDEE are mineralization, biological nitrogen fixation, fertilization and atmospheric nitrogen deposition. Outgoing fluxes are emissions of NO, NO, N and NH, plant nitrogen uptake of NHand NO and leaching. Figure XXX summarizes the mineral nitrogen pools and fluxes considered in ORCHIDEE.
Incoming mineral N fluxes¶
Biological nitrogen fixation (BNF). By default, BNF is not computed into ORCHIDEE and is assumed to be an input data. The BNF input map used currently in ORCHIDEE set the BNF rate as a function of the annual latent heat flux based on the work of Cleveland et al. (1999). It provides BNF rate varying in space but constant in time. In ORCHIDEE, the reactive N produces by BNF feeds the NH pool.
Synthetic fertilization. Synthetic fertilisation is provided through input maps at a yearly time step. It assumes that reactive nitrogen is applied uniformly in time over the year over cropland and grassland area. Several datasets are available with different specificities: information for cropland and grassland, or for C3 and C4 crops only; as total N (to be splitted internally in NO / NH using the parameter RATIO_NH4_FERT) or specifically as NO and NH
Atmospheric N deposition Information on Atmospheric N deposition is updated at a yearly time-step with monthly- or annual-scale data. It provides information on total NO and NH that feed NO and NH pools respectively; or information on subcategories (wet and dry NO and NH)
Outgoing mineral N fluxes¶
Emissions of N2O, NOx, N2 and NH3
Plant nitrogen uptake of NH4 and NO3
Leaching of NH4 and NO3
Internal mineral N fluxes¶
Nitrification
Denitrification
1.9DONE: BVOC emissions¶
ORCHIDEE calculates the emissions of volatile organic compounds (VOCs) from vegetation, also known as biogenic VOCs (BVOCs). BVOCs are generally characterized by a strong chemical reactivity in the atmosphere, together with high natural emissions, and therefore play a key role in the atmospheric chemical composition. Implementing BVOC emission schemes into the land surface model ORCHIDEE enables studying the interactions between the terrestrial biosphere, atmospheric chemistry, and climate. The original emission scheme Lathière et al., 2006 has been extensively updated by Messina et al. (2016), based on the model developed by Guenther et al. (2012). It includes an extended list of biogenic emitted compounds, updated emission factors, and a dependency on light for almost all compounds.
A wide range of BVOCs are simulated, including isoprene, monoterpenes (as a family of compounds and also with eight speciated monoterpenes), sesquiterpenes, methanol, acetone, acetaldehyde, formaldehyde, acetic acid and formic acid. The compounds accounted for by ORCHIDEE solely consist of carbon, hydrogen, and oxygen, hence, there is no interaction between the production of BVOCs in ORCHIDEE and the nitrogen cycle. Moreover, the BVOC emission calculation is a supplementary diagnostic and is not accounted for in the carbon mass balance.
The PFT-specific emission flux (; g C m h) of the specific biogenic compound , at canopy layer is calculated as:
where is the leaf area of canopy layer , is the PFT-specific leaf area (m g, (g C g h) is the PFT-specific basal emission at the leaf level for an individual emitted compound at a reference temperature of 303.15 K and a photosynthetically active radiation of 1000 mol m s. (unitless) is a compound-specific modulator that reflects the deviation from the standard conditions of temperature and photosynthetically active radiation, and (unitless) is a modulator that accounts for the change in emission capacity depending on the age of the leaves.
The temperature and radiation modulator is calculated separately for each compound and canopy layer :
The purpose of is to represent the light-dependent and light-independent processes that lead to emissions. c is the prescribed fraction of emission that is, for one particular BVOC compound, light-dependent, and b are the dependencies of emissions to temperature, for light-dependent and light-independent fractions respectively, and is the radiation-dependency relationship, considered for the light-dependent fraction of emissions. With
where c is a prescribed temperature dependency for each compound , and T is the temperature of the air.
where
and
with c the ideal gas constant, i.e., 8.314 J mol K.
where F is the incoming photosynthetic active radiation in canopy layer calculated following the scheme for direct and diffuse light provided by Spitters et al. (1986) and Spitters (1986). Emissions are thus calculated for each canopy layer considering the sunlit and shaded leaf fractions and the light extinction and light diffusion through canopy. Note that vertical discretisation of the canopy and the light transfer calculated to simulate the BVOC emissions are not necessarily consistent with the canopy discretisation (section 1.5.7) and the light transfer 1.3.2 used in the rest of the model.
The leaf age modulator is calculated for isoprene and methanol only, for which a change in the emission capacity with leaf age has been demonstrated MacDonald & Fall, 1993Guenther et al., 1999 and is equal to unity for other BVOCs. Regarding isoprene and methanol, it is calculated in all leaf age classes, based on the fraction of leaves within each class f calculated in ORCHIDEE (Section 1.5.1, and considering a different emission activity for each class, with a 50% reduction in emissions capacity c for leaf age classes 1 and 4 in the case of isoprene and for leaf age classes 3 and 4 in the case of methanol.
Finally, the total emission per grid cell is obtained by summing over the layer and adding the emission contribution of each individual PFT, weighted by PFT fractional land coverage. Every values of emission factor and light-dependent fraction for each BVOC considered in the model can be found in Messina et al. (2016).
1.10DONE: Vegetation demography¶
Since vegetation canopy acts as the interface between the land and the atmosphere, forest stand structure has implications beyond the carbon and nitrogen budgets of forest management and disturbances, and has been shown to influence albedo, transpiration, photosynthesis, soil temperature, roughness length, and recruitment Amiro et al., 2006Amiro et al., 2006Dore et al., 2010Luyssaert et al., 2014Alkama & Cescatti, 2016Bright et al., 2017. Three demography processes are defined in ORCHIDEE. Succession refers to the aging of vegetation and describes how stands biomass is dynamically allocated to age and circumference classes following growth, management or mortality events. Recruitment refers to cases where small-scale mortality events are compensated by the growth of saplings and happens when management is unmanaged or continuous-cover forestry or in case of a disturbance affecting less than 30% of the stand basal area. Establishment refers to the growth of saplings in an area where all biomass is dead or non-existent, for example after harvest or after a disturbance affecting more than 30% basal area.
1.10.1DONE: Succession within established vegetation¶
To better represent forest succession and its impacts on element, energy, and water fluxes, ORCHIDEE v4.2 distinguishes between different age classes (). Each age class also includes circumference classes to enable simulating forest management, forest disturbances, and more refined simulation of canopy structure. For each PFT, processes such as establishment, phenology, photosynthesis, maintenance respiration, allocation and growth respiration, mortality, land use, disturbances, litter decomposition, and soil decomposition are calculated as described in sections 1.10.3 to 1. Throughout these processes, the multiple circumference classes are considered, with trees in different classes experiencing preferential growth and mortality rates driven by different processes. In long enough simulations or under high mortality conditions, stand density in some circumference classes may eventually drop to zero, reducing the numerical resolution of the carbon and nitrogen allocation scheme (specifically equation 301). When only one populated circumference class remains, the scheme loses its meaning, as all the newly produced biomass is allocated to the sole remaining class. To ensure maintaining the same level of detail throughout the simulation, individuals and their biomass are redistributed across all circumference classes at the end of each day.
The daily redistribution of biomass across circumference classes largely recalculates the structural impact of thinning, disturbances, and recruitment. During the redistribution, individuals are allocated to the circumference classes according to the Weibull distribution, with the shape depending on the forest management strategy and the quadratic mean diameter of the stand for rotational even-aged management (equations 343 to %s). Once the number of individuals in each circumference class is known, their corresponding biomass is moved to the respective class. The new total biomass of each circumference class is then divided by its new number of individuals to obtain the mean biomass of the model tree in each class. With the wood biomass now determined, the circumference of each model tree is calculated, and the boundaries of the circumference classes are updated.
1.10.2DONE: Recruitment within established vegetation¶
Recruitment of saplings within an existing forest PFT compensates for the loss of individuals due to density-driven mortality (section 1.6.3) and other sources of tree mortality. By default, recruitment is calculated once per year and only applies to unmanaged or continuous-cover forestry stands. The number of recruits added each year (; m) in the first age class is given by:
where and are PFT-specific parameters, is the stand density (m) before recruitment, and is the fraction of light reaching the forest floor (equation 358) averaged over the growing season. Recruitment density increases with lower initial stand densities and sparser canopies.
The height of an individual sapling is determined by a PFT-specific parameter (m) and is used to calculate the initial diameter (m) using equation 299. Height and diameter are then used in equation 286 to calculate the sapwood mass (; g plant) for an individual recruit. Subsequently, leaf mass (; g plant) and root mass (; g plant) are calculated using equations 287 and 292, respectively, with the allocation parameters (equation 288) and (equation 293) recalculated to account for the effect of light stress on allocation.
If recruitment occurs outside the growing season in a deciduous PFT, the leaf and root masses are transferred to the reserve pool (; g plant). Finally, the newly recruited biomass and individuals are added to the first circumference class of the existing PFT as follows:
Recruitment is mono-specific and follows the PFT in which it occurs. In other words, ORCHIDEE v4.2 does not explicitly simulate multiple species within a single PFT. However, the PFT parameters can be set to represent a multi-species stand, as in the case of species-rich tropical forests represented by the MTC labelled as Tropical broad-leaf evergreen forest (Table 6).
1.10.3DONE: Establishment of new vegetation¶
In ORCHIDEE v4.2, new vegetation is established following a stand-replacing event, which can be natural mortality (section 1.6), natural disturbance (section 1), land cover change (section 1.1), forest management (section 1.4), or crop harvesting (section 1.3). In cases of land cover change (section 1.1), the newly established vegetation has a different PFT than that of the previous vegetation. Similarly, for a change in forest PFT (section
), the newly established vegetation may have a different PFT. In all other cases, the newly established vegetation retains the same PFT as the previous vegetation.
The fraction of the newly established PFT within the grid cell (; unitless) is read from an external annual vegetation distribution map (section 1.2) or derived from internal areal bookkeeping in the case of natural disturbances (section 1.6). For forests, the size distribution of saplings across circumferences classes in the first age class is calculated, including the diameter (; m), height (; m) and biomass of model individuals (; g plant) as well as the number of saplings in each circumference class. The approach formalized in ORCHIDEE v4.2 introduces a circular dependency because the density distribution across circumference classes depends on the quadratic mean diameter (section 1.5.5), while the quadratic mean diameter itself depends on the density distribution (section %s). This circularity is resolved through a single iteration that begins by calculating the representative diameter for each circumference class using the PFT-specific minimum diameter (; m) and maximum diameter (; m):
An initial estimate of the frequency distribution of individuals in each circumference class is derived from the Weibull distribution (equations 344 to %s) with distribution parameters depending on PFT and management strategy as the central circumference class diameter, the diameter at which the continuous Weibull distribution is truncated ( in equation 344) depending on the forest management strategy and the shape (; unitless) parameter of the Weibull distribution is either calculated or prescribed depending on the forest management strategy (equation 343).
The total number of individuals is determined using equation 339 with the quadratic mean diameter (; m). The frequency distribution in each circ class and total number of individuals (; m) are then used to calculate the number of individuals in each circumference class .
The representative diameters in each circumference class derived from the Weibull distribution are also used to calculate the biomass for individual trees in each circumference class following the same allocation principles (section 1.4.2). The diameter of the representative sapling in each circumference class is determined using equation 403 and it is then used to calculate the corresponding tree height via the allometric equation 299. Height and diameter are subsequently used in equation 286 to calculate the sapwood mass (; g plant) of an individual sapling. Then, leaf mass (; g plant) and root mass (; g plant) of a sapling are calculated using Equations 287 and 292, respectively, where the allocation parameters (equation 288) and (equation 293) are calculated assuming that there is no light stress at the time of vegetation establishment. For deciduous PFTs, if recruitment occurs outside the growing season, leaf and root masses are moved into the reserve pool (; g plant). Since the allocation equations and the self-thinning relationship are not suited for seedlings, initial diameters (i.e., and ) are prescribed to exceed 0.02 m. The initial forests thus better represent the sapling than the seedling stage.
For grassland and cropland PFTs, the stand density is prescribed and fixed. These PFTs are initialized by prescribing the initial height, which is converted into a leaf area using a single prescribed parameter. After calculating the allocation parameters (equation 288), (equation 293), and the specific leaf area (equation 1.5.2), leaf mass (; g plant) is computed as follows:
With the leaf mass and allocation parameters determined, root (; g plant) and stem mass (; g plant) are calculated using Equations 287 and 292. All grassland and cropland PFTs are assumed to be deciduous, with crops established on the day of bud break. As with forest vegetation, if the grassland PFT is established outside the growing season, the leaf and root mass are transferred into the reserve pool (; g plant) until bud break.
During vegetation establishment, the carbon necessary to build the initial biomass is taken from the atmosphere. Nitrogen needed for establishment is drawn from the soil mineral nitrogen pool, which is replenished through mineralisation (section 1.8.5), atmospheric deposition and nitrogen fertilisation (section 1.9). If the mineral nitrogen pool cannot satisfy the initial demand, nitrogen is supplied from the organic soil nitrogen pools (section 1.8.1). If the latter pools cannot meet the demand either, nitrogen is finally taken from the atmosphere.
- Lieth, H. (1974). Phenology and seasonality modeling (Vol. 8). Springer.
- Piao, S., Liu, Q., Chen, A., Janssens, I. A., Fu, Y., Dai, J., Liu, L., Lian, X., Shen, M., & Zhu, X. (2019). Plant phenology and global climate change: Current progresses and challenges. Global Change Biology, 25(6), 1922–1940. 10.1111/gcb.14619
- Botta, A., Viovy, N., Ciais, P., Friedlingstein, P., & Monfray, P. (2000). A global prognostic scheme of leaf onset using satellite data. Global Change Biology, 6(7), 709–725. 10.1046/j.1365-2486.2000.00362.x
- Chuine, I. (2000). A unified model for budburst of trees. Journal of Theoretical Biology, 207(3), 337–347. 10.1006/jtbi.2000.2178
- Hänninen, H., & Kramer, K. (2007). A framework for modelling the annual cycle of trees in boreal and temperate regions. Silva Fennica, 41(1), 167–205. 10.14214/sf.313
- Orlandi, F., Garcia-Mozo, H., Vazquez Ezquerra, L., Romano, B., Dominguez, E., Galan, C., & Fornaciari, M. (2004). Phenological olive chilling requirements in Umbria (Italy) and Andalusia (Spain). Plant Biosystems, 138(2), 111–116. 10.1080/11263500412331283762
- Cannell, M. G. R., & Smith, R. I. (1986). Climatic Warming, Spring Budburst and Forest Damage on Trees. The Journal of Applied Ecology, 23(1), 177. 10.2307/2403090
- Murray, M. B., Cannell, M. G. R., & Smith, R. I. (1989). Date of Budburst of Fifteen Tree Species in Britain Following Climatic Warming. The Journal of Applied Ecology, 26(2), 693. 10.2307/2404093
- Krinner, G., Nicolas, V., de Noblet-Ducoudre, N., Ogée, J., Polcher, J., Friedlingstein, P., Ciais, P., Sitch, S., & Prentice, I. C. (2005). A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Global Biogeochemical Cycles, 19(1), GB1015. 10.1029/2003GB002199
- MacBean, N., Maignan, F., Peylin, P., Bacour, C., Bréon, F.-M., & Ciais, P. (2015). Using satellite data to improve the leaf phenology of a global terrestrial biosphere model. Biogeosciences, 12(23), 7185–7208.
- Yin, X., & Struik, P. C. (2009). C3 and C4 photosynthesis models: An overview from the perspective of crop modelling. NJAS - Wageningen Journal of Life Sciences, 57(1), 27–38. 10.1016/j.njas.2009.07.001
- Farquhar, G., & Wong, S. (1984). An empirical model of stomatal conductance. Functional Plant Biology, 11(3), 191–210.
- Medlyn, B. E., Dreyer, E., Ellsworth, D., Forstreuter, M., Harley, P. C., Kirschbaum, M. U. F., Le Roux, X., Montpied, P., Strassemeyer, J., Walcroft, A., Wang, K., & Loustau, D. (2002). Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant, Cell and Environment, 25(9), 1167–1179. 10.1046/j.1365-3040.2002.00891.x
- Amthor, J. S. (1984). The role of maintenance respiration in plant growth. Plant, Cell and Environment, 7(8), 561–569. 10.1111/1365-3040.ep11591833
- Vicca, S., Luyssaert, S., Peñuelas, J., Campioli, M., Chapin, F. S., Ciais, P., Heinemeyer, A., Högberg, P., Kutsch, W. L., Law, B. E., Malhi, Y., Papale, D., Piao, S. L., Reichstein, M., Schulze, E. D., & Janssens, I. A. (2012). Fertile forests produce biomass more efficiently. Ecology Letters, 15(6), 520–526. 10.1111/j.1461-0248.2012.01775.x